Project Overview
Hydrogen (H2) is the most abundant molecule in the universe, and its interaction with surfaces plays a pivotal role in many applications, from the formation of stars and the safe storage of rocket fuel, to hydrogen fuel cells and industrial catalysis. Despite also being the simplest molecule in existence, we do not yet have an accurate predictive understanding of the interaction of H2 with even the simplest solid surfaces. As such, to improve our knowledge and understanding, carefully controlled experiments are required which probe the collisions of H2 with surfaces at a fundamental, molecular level. There are several factors that can determine the outcome of these collisions, including how fast the molecule is travelling and how it is rotating with respect to the surface, as well as the material the surface is made from and the surface temperature. Experiments which can independently vary each of these (and other) factors will provide the most detailed insight into this gas-surface collision, as they probe the role each factor plays, remove the need for averaging and the uncertainty this leads to, and provide the most stringent tests of theoretical models that must accurately reproduce these carefully controlled experiments.
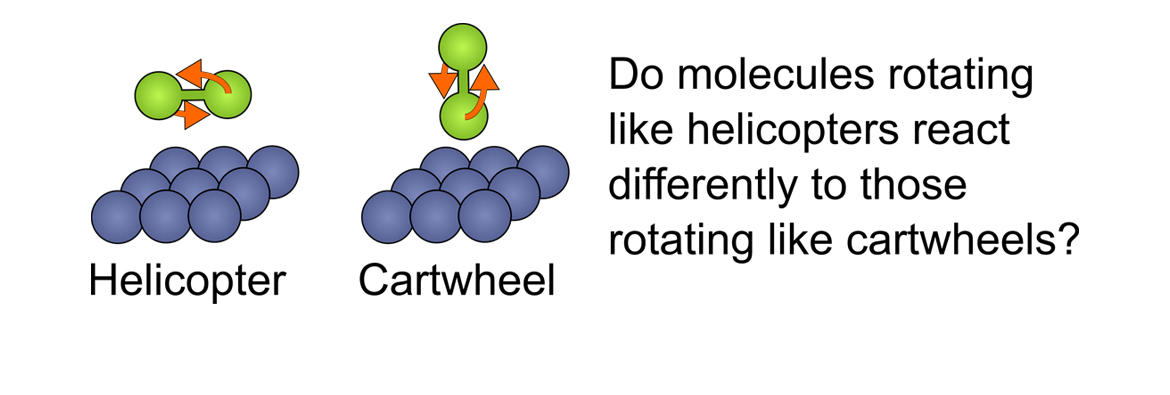
In this project the rotational orientation of hydrogen molecules, which can be considered to correspond to whether the molecule is rotating like a helicopter or a cartwheel, will be controlled and manipulated to determine whether this parameter can be used to control the reactivity of H2 in collisions with a surface. These measurements will be performed using a unique and novel magnetic manipulation experimental apparatus and technique, to control the rotational orientation of H2 molecules in scattering experiments. The results of these experiments will provide the first quantitative insight into the effect the rotational orientation of H2 has on the probability the molecule dissociates when it collides with a surface. The project will also explore how the rotational orientation of H2 changes the transfer of energy between the molecule and the surface. This energy transfer process is an important step for trapping a molecule on a surface and correspondingly changes reaction probabilities, yet its relation to the orientation of the rotating molecule was so far inaccessible to previous experiments.